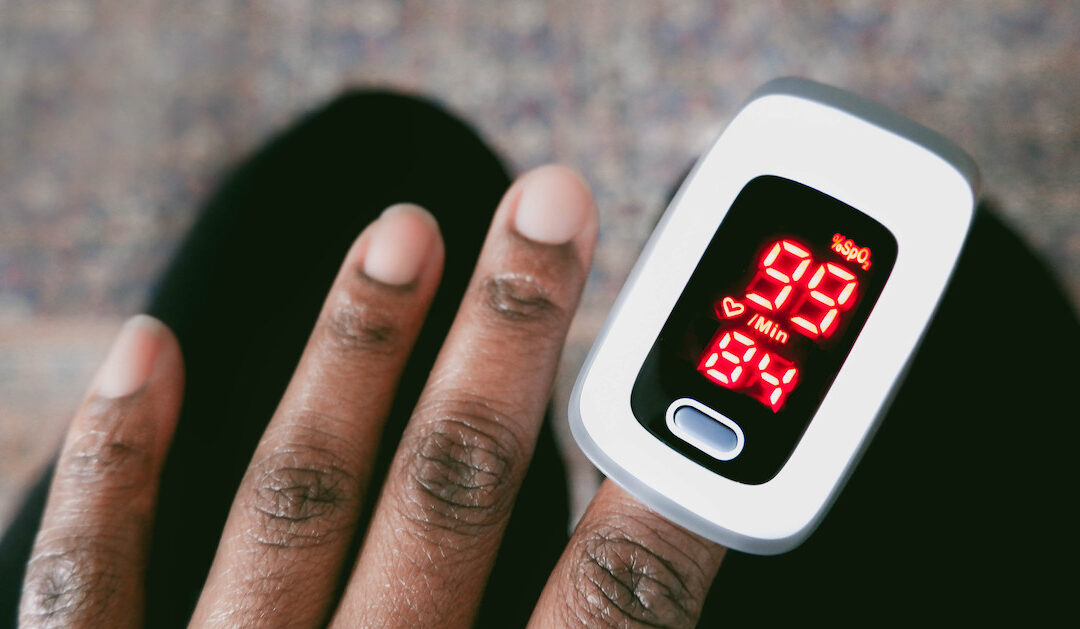
The U.S. Food and Drug Administration has published updated draft recommendations to help improve the performance of pulse oximeters across skin tones.
The U.S. Food and Drug Administration has published updated draft recommendations to help improve the performance of pulse oximeters across skin tones. Clinical, Compliance & Legal, Government & Policy, Medical Devices, Population Health Most Popular News from healthcareitnews.comRead More

 Add to favorites
Add to favorites